ca orbital diagram
Orbital diagrams use the same basic format but instead of numbers for the electrons they use and arrows as well as giving each orbital its own line to represent the. Every person should learn about the chemical.
:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Nsbpjvcwhj2bxm
Calcium has atomic number of 20 so it has total of 20 electrons which should.

. Are you searching for Calcium Electron Configuration Ca with an Orbital Diagram. Science Chemistry QA Library QUESTION 49 Draw the molecular orbital diagram and determine the bond order for Ca 2 2. In writing the electron configuration for Calcium the first two electrons will go in.
8 - Drawing Molecular Orbital Diagrams. Which has been discussed in detail above. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below.
To write the orbital diagram for the Calcium atom Ca first we need to write the electron configuration for just Ca. 1s is the closest and lowest energy. The Pauli Exclusion Principle - This says that only two electrons can stay in a single orbital.
Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already. Interactive Periodic Table Let me tell you how this Interactive. Get Ca Orbital Diagram MP3 Download 309 MB on Navidbiglarimusic Quick and Easy - NAVID BIGLARI MUSIC How To Write The Orbital Diagram For Calcium Ca 0215 min 320 kbps 309.
In an orbital filling diagram the individual orbitals are shown. However the molecular orbital diagram we see in Figure 925 Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 5-7 can be used to. The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom it uses an arrow to represent the electrons every orbital one box contains a maximum.
Hunds rule - This states that electrons enter different orbitals in the same sublevel before. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. When we write the configuration well put all 20 electrons in orbitals around the nucleus of the Calcium atom.
To do that we need to find the number of electrons for the. QUESTION 49 Draw the molecular orbital diagram and. To write the orbital diagram of chromium Cr you have to do the electron configuration of chromium.
Part B Enter an orbital diagram for Ca Drag the appropriate. July 16 2022 by Sneha Leave a Comment. View the full answer Transcribed image text.
The atomic number of an element is the number of electrons and protons in that element. Calcium orbital diagram According to Hunds principle the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Free Gift for you.
That is the number of electrons and protons in the sodium atom is eleven.

Write The Electron Configuration And Orbital Diagrams Of Be 2 Al 3 Ca Homework Study Com

Delocalized Bonding And Molecular Orbitals

Orbital Diagrams Ppt Download

Draw An Orbital Diagram And Lewis Structure For A Li And S Homework Study Com

Orbital Notation Watch After Electron Configuration Science Chemistry Showme

Fluorine Orbital Diagram Electron Configuration And Valence Electron
The Order Of Filling 3d And 4s Orbitals Chemistry Libretexts
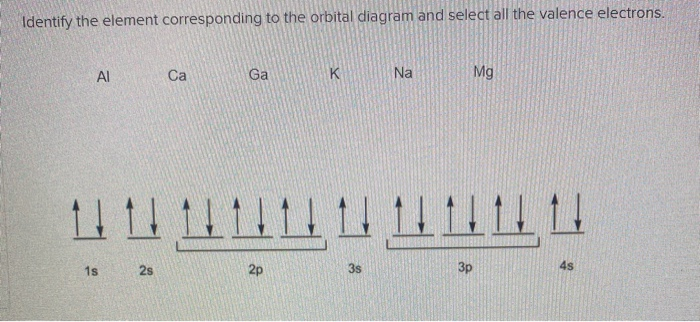
Solved Identify The Element Corresponding To The Orbital Chegg Com
Molecular Structure Atomic Orbitals

Orbital Diagram Calculator Get Instant Answer

Draw The Orbital Diagram Ca Ion And State The Number Of Three Fundamental Particles Present In It
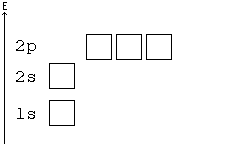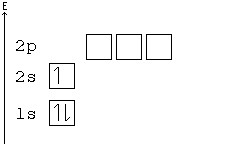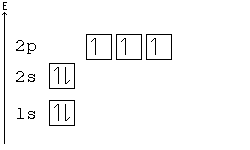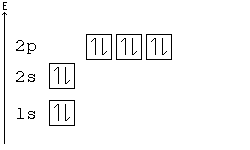
General Chemistry Filling Electron Shells Wikibooks Open Books For An Open World
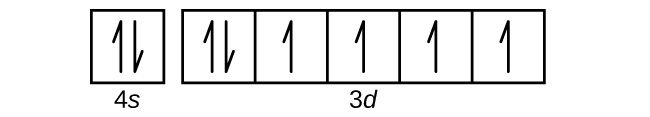
Electronic Structure Of Atoms Electron Configurations Atoms First Openstax
Quantum Mechanics

Write The Abbreviated Orbital Diagrams For The Following Elements And State Whether They Are Paramagnetic Or Diamagnetic A Ni 2 B Ca 2 Homework Study Com

Draw An Orbital Diagram For Al Electrons And Ions Which Electrons Are Responsible For Chemical Properties Valence Electrons Core Electrons Ppt Download
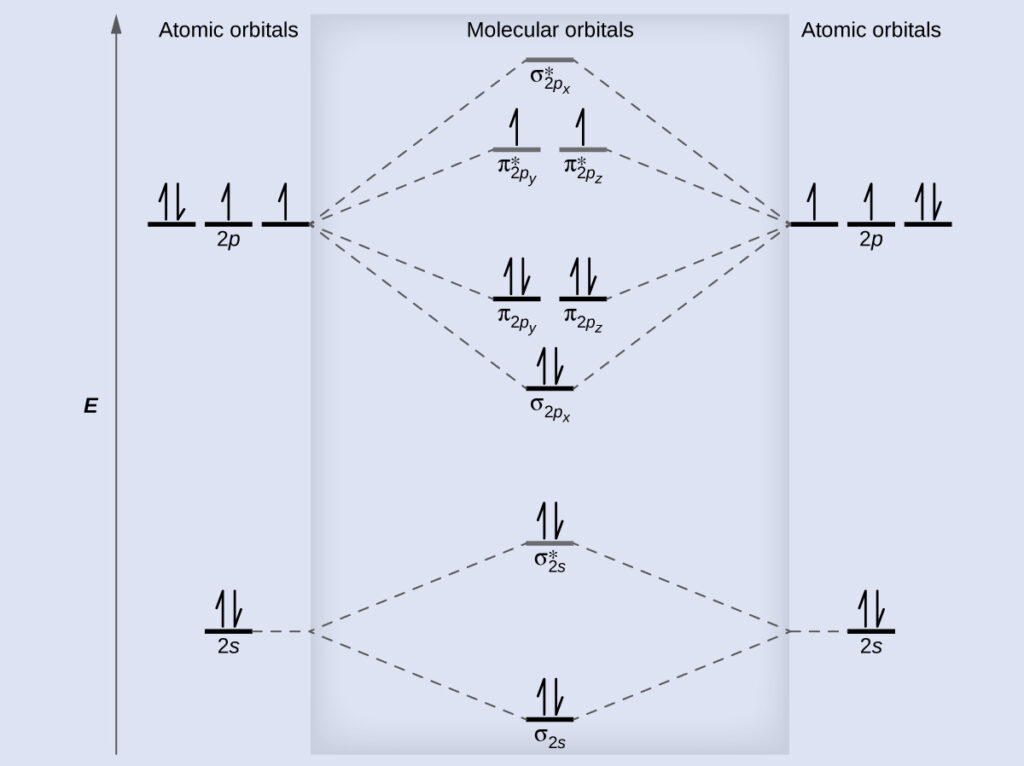
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Ucalgary Chem Textbook